icotec
The Spine Tumor Company
We Help Patients
The world’s first spinal implants made of nonmetallic and radiolucent BlackArmor® enables a full spectrum of treatment modalities in adjuvant tumor therapy and enhances postoperative imaging diagnostics.
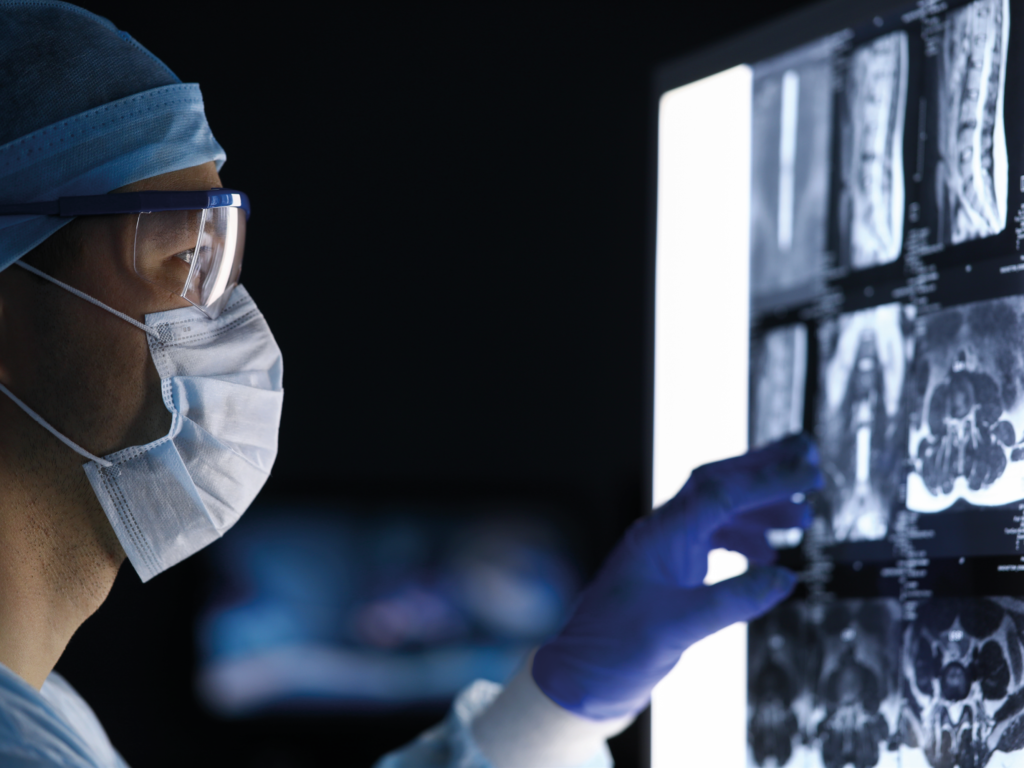
Learn How BlackArmor® Implants Improve the Treatment of Patients with Spinal Neoplasms
Crucial benefits along the entire treatment path: optimized radiation therapy, expanded diagnostic and therapeutic options, and reliable follow-up.
CuttingEdge Technology in the Fight against Spinal Tumors
BlackArmor® Carbon/PEEK from icotec: the ideal material for optimizing care for patients with spinal neoplasms


Literature
Discover more information about our products, browse our
brochures and flyers, and explore links to scientific publications.



