Material Matters: Choosing the Right Implant for Spinal Tumor Patients
Conventional metallic implants, such as titanium, are not radiolucent and cause artifacts during postoperative radiographic imaging (CTs, MRIs, etc.), limiting diagnostic and therapeutic options. BlackArmor® Carbon/PEEK, icotec’s radiolucent material, is artifact-free, which improves postoperative imaging1Ringel et al. (2017): Radiolucent Carbon Fiber-Reinforced Pedicle Screws for Treatment of Spinal Tumors: Advantages for Radiation Planning and Follow-Up Imaging. World Neurosurgery. (PubMed, PMID: 28478252)and makes a crucial contribution to optimized spinal tumor and metastasis therapy.2Poel et al. (2020): Assessing the Advantages of CFR-PEEK over Titanium Spinal Stabilization Implants in Proton Therapy – A Phantom Study. Physics in Medicine and Biology. (PubMed, PMID: 32315991)3Müller et al. (2020): The Dosimetric Impact of Stabilizing Spinal Implants in Radiotherapy Treatment Planning with Protons and Photons: Standard Titanium Alloy vs. Radiolucent Carbon-Fiber-Reinforced PEEK Systems. Journal of Applied Clinical Medical Physics. (PubMed, PMID: 32476247)4Shi et al. (2022): Comprehensive Evaluation of Carbon-Fiber-Reinforced Polyetheretherketone (CFR-PEEK) Spinal Hardware for Proton and Photon Planning. Technology in Cancer Research & Treatment. (PubMed, PMID: 35410544) This makes perioperative decision-making in the event of symptoms easier.5Fleege et al. (2020): Carbon-Fiber-Reinforced Pedicle Screws Reduce Artifacts in Magnetic Resonance Imaging of Patients with Lumbar Spondylodesis. Scientific Reports. (PubMed, PMID: 32999385) In adjuvant radiation therapy, BlackArmor® Carbon/PEEK improves local tumor control and reduces toxicity to organs at risk.2Poel et al. (2020): Assessing the Advantages of CFR-PEEK over Titanium Spinal Stabilization Implants in Proton Therapy – A Phantom Study. Physics in Medicine and Biology. (PubMed, PMID: 32315991)4Shi et al. (2022): Comprehensive Evaluation of Carbon-Fiber-Reinforced Polyetheretherketone (CFR-PEEK) Spinal Hardware for Proton and Photon Planning. Technology in Cancer Research & Treatment. (PubMed, PMID: 35410544)6Krätzig et al. (2021): Carbon-Fiber-Reinforced PEEK versus Titanium Implants: An In Vitro Comparison of Susceptibility Artifacts in CT and MR Imaging. Neurosurgical Review. (PubMed, PMID: 32930911)It also makes earlier detection of local recurrences more likely.7Almeida et al. (2022): Carbon Fiber Reinforced PEEK Implants for Spine Tumors: A Single Center Experience. The Spine Journal, Volume 22, Issue 9, Supplement, Page S185 (Link)As a result, there are compelling clinical benefits for surgical treatment, radiation therapy, and aftercare. A patient-centered, multidisciplinary treatment approach improves the care journey for the patient.
“Successful treatment of spinal tumors is based on precise coordination of the individual therapy steps as well as close interdisciplinary cooperation. Carbon/PEEK implants support every phase of this complex process.”
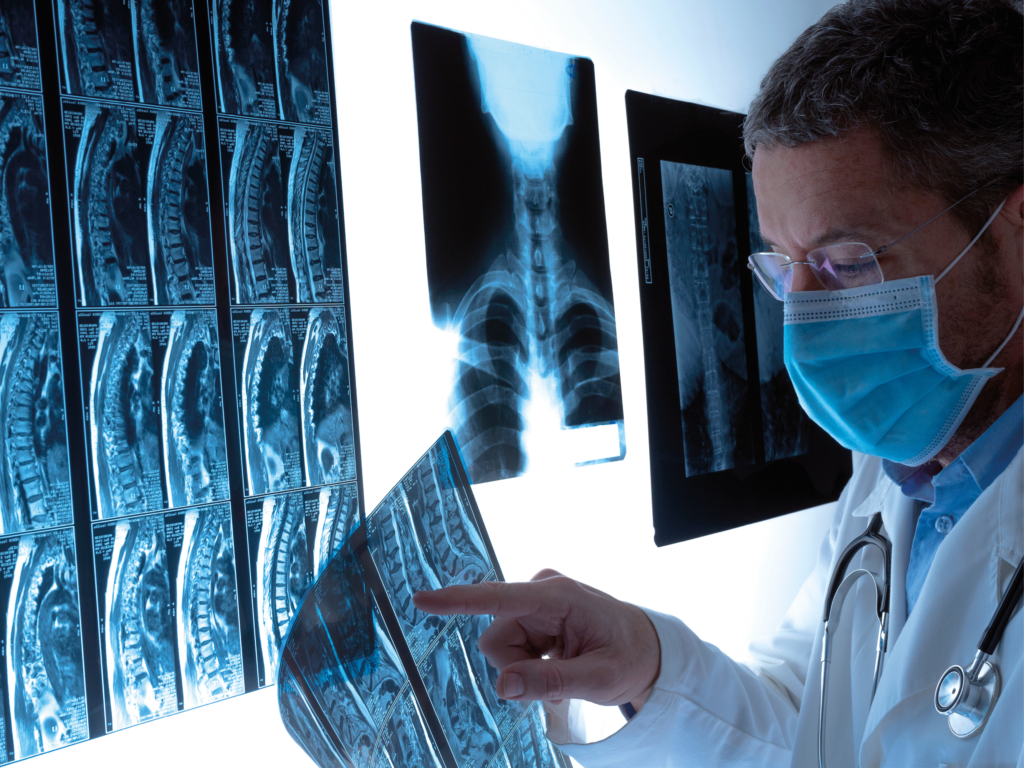
Surgery
Optimize perioperative management by using the artifact-free BlackArmor® Carbon/PEEK.
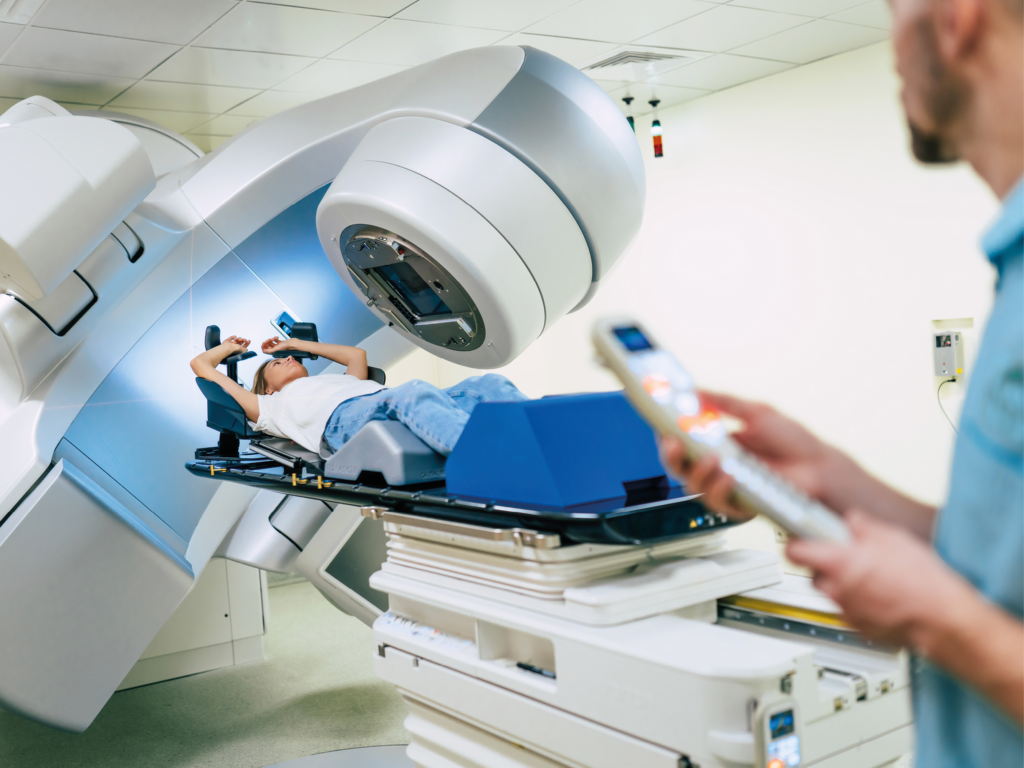
Radiation Therapy
Improve radiooncological treatment and enable safer care with the radiolucent and artifact-free BlackArmor® Carbon/PEEK.