"70% of spinal metastases occur in the thoracic spine. We are extremely proud that icotec is the world-leading provider of extended solutions for the stabilization of this frequently affected spinal region with the extension of its VADER® product family with cannulated and fenestrated Ø 4.5 mm pedicle screws and longer, multi-curved rod options. CE approval under the latest MDR regulations represents an important milestone in our continued commitment to provide patients in need access to spinal implants made from radiolucent BlackArmor®, allowing for expanded treatment options and safer tumor care."
August 22, 2025 – Altstätten, Switzerland – icotec ag, the leading medical device manufacturer of Carbon/PEEK spinal implants, announces the CE approval under the European Medical Device Regulation (MDR) to market the VADER® Pedicle System inclusive of Ø 4.5 mm pedicle screws and long Carbon/PEEK rods made from BlackArmor® and its use with Medtronic StealthStation™ navigation system.
The extended VADER® Pedicle System maintains its proven indication and versatility for open, MIS and cement-augmented surgical techniques while providing additional options for stabilizing the upper-thoracic region of the spine.
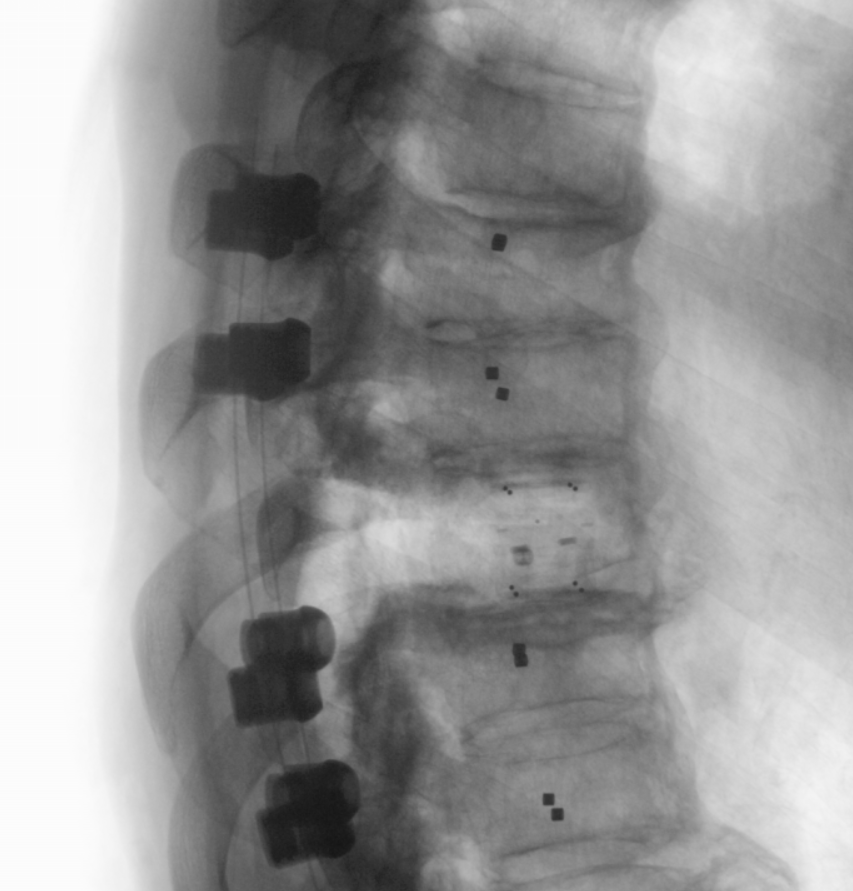
Radiolucent BlackArmor® implants broaden therapeutic options for patients with spinal tumors. Lateral X-ray of the thoracic spine, stabilized with VADER® Pedicle System and KONG®-TL VBR
The VADER® Pedicle System now consists of pedicle screws of diameters between 4.5 and 7.5 mm in lengths of 25 to 60 mm, and rods in different shapes up to 300 mm in length. VADER® screws and rods are made of unique BlackArmor® Carbon/PEEK composite material. BlackArmor® offers excellent stabilization of the spine while being completely radiolucent, allowing for clear visualization in postoperative imaging, improved radiation planning and dose delivery, and expanding treatment options for patients with spinal tumors.
The introduction of extended-length rods with embedded titanium wires allows surgeons to intraoperatively select from multiple curvature options and precisely customize the rod length using a dedicated cutting device — providing greater flexibility and efficiency during spinal fixation procedures.
In addition, the VADER® Pedicle System has received CE certification under MDR for its use with the Medtronic StealthStation™ navigation system, which enables seamless integration into navigated spinal procedures, enhancing intraoperative precision, surgical workflow efficiency, and reduced intraoperative radiation exposure to the patient and surgical team.
About icotec ag
icotec is the leading company for the treatment of spinal tumors and spinal infections with a new generation of high-tech implants. With its BlackArmor® Carbon/PEEK implant, icotec combines cutting-edge technologies and industry expertise to deliver innovative and reliable solutions for spine surgeons and their patients and is dedicated to advancing the field of spinal implantation. With a track record of clinical success and a commitment to continuous innovation, icotec is poised to shape the future of spinal surgery. The comprehensive product portfolio has received FDA clearance and is supported by numerous Key Opinion Leaders and Cancer Therapy Centers worldwide. More information can be found at www.icotec-medical.com.
Contact
For more information or questions, please contact hello@icotec-medical.com.